Molecular determinants of cardiac remodelling and heart failure
Team 2 – UMR1167 – Inserm – Lille University – CHU Lille – Institut Pasteur de Lille
Presentation
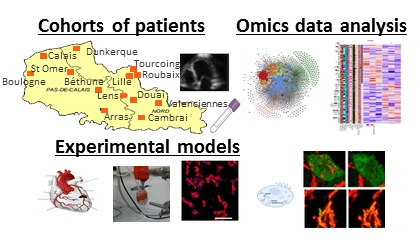
Our team “Molecular Determinants of Remodeling and Heart Failure” is composed of researchers, clinical university hospital researchers, post-doctoral researchers, engineers, technicians and students. We are developing translational research from the patient’s bedside to the bench. We develop and use advanced techniques in experimental medicine (cell and animal models), proteomics and bioinformatics.
Objectives of the team: To find new biomarkers for the diagnosis and prognosis of cardiac remodeling after myocardial infarction and heart failure and to characterize the pathophysiological mechanisms by developing translational research at the interface between the cardiologic hospital and the laboratory.
(1) To identify circulating biomarkers of cardiac remodeling and heart failure through novel approaches to systematic screening ;
(2) To clinically validate the identified biomarkers ;
(3) To identify and decipher the pathophysiological mechanisms of targets identified as being involved in heart failure.
Highlights
- Dotation Fédération Française de Cardiologie.
Prediction of cardiac remodeling by high throughput proteomics (CHU de Lille, Inserm, Univ Lille, Inria) (2020-2021).
The objective of this project is to use a high throughput proteomic technique that allows the determination of more than 5000 proteins in a single plasma sample (SOMASCAN technique) to predict remodeling after myocardial infarction. The assays were performed in plasma samples from patients included in the REVE and REVE-2 studies. The perspectives in terms of research and public health are a better stratification of the risk of heart failure after infarction and also a better physiopathological knowledge that could allow new therapeutic research.
- Dotation Fédération Française de Cardiologie (Paris, Créteil, Lille).
Role of the PrPc protein in the pathophysiology of left ventricular remodeling after myocardial infarction (2021-2022).
The objectives of this project are 1) to clarify the role of PrPc in post-infarction left ventricular remodeling, 2) to evaluate the potential of PrPc as a therapeutic target, 3) to refine the predictive value of PrPc blood levels in the prediction of left ventricular remodeling and the occurrence of clinical features.
- FHU-CARNAVAL: CArdiac Research Network on Aortic Valve and heart failure (Caen-Rouen-Amiens-Lille (2021-2025).
The CARNAVAL project aims 1) to better understand the pathophysiology of heart failure and aortic stenosis, 2) to identify new therapeutic targets based on preclinical results, 3) to evaluate new methods of data analysis, 4) to propose new methods of management, 5) to address the question of the care pathway of a TAVI-treated patient, 6) to formulate and answer ethical questions about innovative techniques, 7) to propose new and original training courses and to conduct public awareness actions.
Transversal projects
 Within the framework of the CPER Longevity, we developed a collaboration with the CIIL teams (M Pichavant, I Wolowczuk, C Grangette, P Brodin) which allowed us to have two projects financed (CONNECTING and COMMONLY).
Within the framework of the CPER Longevity, we developed a collaboration with the CIIL teams (M Pichavant, I Wolowczuk, C Grangette, P Brodin) which allowed us to have two projects financed (CONNECTING and COMMONLY).
The CONNECTING project (2017-2018) aims to understand how the association “consumption of a high-fat diet and chronic exposure to cigarette smoke” can have deleterious effects on the cardiovascular, pulmonary, metabolic and intestinal compartments. This project aims to understand the cellular and molecular mechanisms involved in the observed deleterious effects.
The COMMONLY project (2019-2020) aims to investigate the role of oxidative stress in the deleterious effects of the high-fat diet associated with cigarette smoke. The role of the immunosensitive gene 1 (IRG1) – a factor involved in the modulation of reactive oxygen species and the production of itaconic acid – in the adverse health effects of co-exposure to cigarette smoking and a high-fat diet will be studied at the whole body and tissue (lungs, adipose tissue, heart and intestine) level.
 Within the framework of the CPER Longevity, we developed a collaboration with the team of Eric Boulanger allowing to have the project RAGING granted in 2018-2020 (IPL – UdL – HEI).
Within the framework of the CPER Longevity, we developed a collaboration with the team of Eric Boulanger allowing to have the project RAGING granted in 2018-2020 (IPL – UdL – HEI).
Inflammaging, a contraction of the terms “inflammation” and “aging”, corresponds to a low-grade state of inflammation, diffuse in the body, progressing slowly, with no obvious inflammatory focus. This systemic inflammation constitutes the substrate common to most chronic diseases linked to aging (Alzheimer’s disease, Parkinson’s disease, atherosclerosis, cardiovascular diseases, AMD, type II diabetes, osteoporosis, cancer, etc.) Inflammaging poses a real health problem public, since it not only increases morbidity and mortality, but also strongly impacts the quality of life of patients. Consequently, the identification of the biological processes making it possible to control it is essential to allow the population to “age better”. The objectives of the project are to design, select, develop and test (in vitro / in vivo) RAGE antagonists to assess the global role of RAGE in inflammaging. The project is divided into 2 main tasks :
- Develop RAGE antagonists
- Biologically evaluate the antagonists developed
Members
Florence PINET
DR1 Inserm, group leader
ORCID number : 0000-0002-5471-1487
Christophe BAUTERS
PU-PH Univ Lille/ CHU Lille
ORCID number : 0000-0001-8268-8467
Pascal DEGROOTE
PH CHU de Lille
ORCID number : 0000-0002-6211-0147
Nicolas LAMBLIN
PU-PH Univ Lille/ CHU Lille
ORCID number : 0000-0003-3754-1241
Olivia BESEME
Engineer, Univ Lille
Maggy CHWASTYNIAK
Technician IPL
Émilie DUBOIS-DERUY
Post-doc
Annie TURKIEH
Post-doc
Fadi ABOU ZEID
PhD student
Wilfried HEYSE
PhD student
Victoriane PEUGNET
PhD student
Yara EL MASRI
Master student
Fréderic NAVEZ
Master student
Julian LALOYAUX
License student
Fabien DWORNIKOWSKI
License student
Guillaume MACHADO
Licence student
Publications
Gupta SK, Foinquinos A, Thum S, Remke J, Zimmer K, Bauters C, de Groote P, Boon R, de Windt L, Preissl S, Hein L, Batkai S, Pinet F, Thum T (2016).
Preclinical development of a microRNA-based therapy for the infarcted heart of elderly individuals.
J Am Coll Cardiol 66:1557-71. doi: 10.1016/j.jacc.2016.07.739.
Turkieh A, Fertin M, Bouvet M, Mulder P, Drobecq H, Lemesle G, Lamblin N, de Groote P, Porouchani S, Chwastyniak M, Beseme O, Amouyel P, Mouquet F, Balligand JL, Richard V, Bauters C, Pinet F (2018).
Expression and implication of clusterin in left ventricular remodeling after myocardial infarction.
Circ- Heart Fail. 11(6):e004838. doi: 10.1161/CIRCHEARTFAILURE.117.004838.
Turkieh A, Porouchani S, Beseme O, Chwastyniak M, Amouyel P, Lamblin N, Balligand JL, Bauters C, Pinet F (2019).
Increased clusterin levels after myocardial infarction protects cardiomyocytes from apoptosis induced by a defect in protein degradation systems activity.
Cell Death Dis. 10(8):608. doi: 10.1038/s41419-019-1857-x.
Cuvellier M, Vandewalle V, Brunin M, Beseme O, Hulot A, de Groote P, Amouyel P, Bauters C, Marot G, Pinet F. (2019).
Circulating proteomic signature of early death in heart failure patients with reduced ejection fraction.
Sci Rep Dec 16;9(1):19202. doi: 10.1038/s41598-019-55727-1.
Perdomo L, Vergori L, Duluc L, Vidal-Gomez X, Soleti R, Chwastyniak M, Bisserier M, Le Lay S, Villard A, Simard G, Meilhac O, Lezoualc’h F, Khantalin I, Veerapen R, Dubois S, Boursier J, Henni S, Gagnadoux F, Pinet F, Andriantsitohaina R, Martinez MC. (2020).
Microvesicles-associated RAP1 accumulates in atherosclerotic plaques, correlates with vascular risks and mediates early steps of atherosclerosis.
Circ Res Aug 28;127(6):747-760. doi: 10.1161/CIRCRESAHA.120.317086.
Keywords
Heart failure ; Oxidative stress ; Biomarkers ; Proteomic