Glycation : from inflammation to aging
Team 5 – UMR1167 – Université de Lille – INSERM – CHU Lille – Institut Pasteur de Lille, JUNIA

Presentation
Our team is studying glycation and its involvement in inflammation leading to aging. AGEs (Advanced Glycation End-Products) are formed during diabetes, kidney failure, inflammation and aging (endogenous AGEs). AGEs are also formed during the high temperature sterilization of glucose solutions and during cooking of foods (exogenous AGEs). AGEs, irreversibly formed by the Maillard reaction, result from the non-enzymatic binding of a sugar to a protein. AGEs exert their toxicity by 3 mechanisms: in situ glycation, AGE deposits and interaction with RAGE, AGEs receptor.
Our team’s project (Fig. 1) consists of 3 steps
- Dissecting the metabolic impact of glycation on low grade inflammation and its consequences on aging ;
- Study the RAGE-Ligand axis in acute and chronic inflammation leading to aging ;
- Develop antagonist drugs for RAGE to control inflammaging: aging related to low inflammation linked to advancing age.
Highlights
- We have demonstrated that the activation of the Ligands / RAGE axis is responsible for signal transduction leading to an acute or chronic pro-inflammatory state with low grade depending on the context. This low-grade inflammation accelerates aging, especially renal aging (interstitial fibrosis, tubular atrophy and glomerulosclerosis) and leads to what is grouped together under the term inflammaging (M Frimat et al, Aging, 2019).
- We have shown in mice (wild type and db/db) that a twelve-week treatment with a probiotic strain of Lactobacillus improves certain characteristics of diabetes: reduced levels of early markers (product of Amadori – furosin) and advanced (carboxymethyllysine) glycation in the kidneys, and improved levels of plasma lipid profiles. (A Guilbaud et al, Mol Nut Food Res, 2020).
- In order to better understand the aging mechanisms induced by the activation of the RAGE-Ligands axis, we have developed in the laboratory and used the model of aging Caenorhabditis elegans. Our first results validate the use of C. elegans as an animal model to demonstrate the molecular mechanisms involved in the toxicity of AGEs and the activation of RAGE.
- We demonstrated that a diet enriched in AGEs accelerates endothelial dysfunction and vascular aging (arterial stiffness) via RAGE in mice (N Grossin et al, Mol Nut Food Res, 2015).
- We are developping an analytical procedure by liquid chromatography coupled to mass spectrometry to quantify biomarkers of non-enzymatic, post-translational modifications (glycation, carbamylation and oxidation) of proteins in different matrices.
Transversal projects
 CPER RAGING 2018-2020 (IPL – UdL – HEI)
CPER RAGING 2018-2020 (IPL – UdL – HEI)
Inflammaging, a contraction of the terms “inflammation” and “aging”, corresponds to a low-grade state of inflammation, diffuse in the body, progressing slowly, with no obvious inflammatory focus. This systemic inflammation constitutes the substrate common to most chronic diseases linked to aging (Alzheimer’s disease, Parkinson’s disease, atherosclerosis, cardiovascular diseases, AMD, type II diabetes, osteoporosis, cancer, etc.) Inflammaging poses a real health problem public, since it not only increases morbidity and mortality, but also strongly impacts the quality of life of patients. Consequently, the identification of the biological processes making it possible to control it is essential to allow the population to “age better”. The objectives of the project are to design, select, develop and test (in vitro / in vivo) RAGE antagonists to assess the global role of RAGE in inflammaging.
The project is divided into 2 main tasks:
1- Develop RAGE antagonists
2- Biologically evaluate the antagonists developed
 ANR ExoAGEing 2020-2023 (UdLille – HEI– LaSalle Beauvais – UdReims)
ANR ExoAGEing 2020-2023 (UdLille – HEI– LaSalle Beauvais – UdReims)
Advanced Glycation End-products (AGEs), notably carboxymethyl-lysine (CML), are involved in age-related diseases and found at high levels in several processed foods. The exposure to dietary AGES, particularly in critical developmental periods but also throughout life, raises questions about their harmfulness to health, specifically their role in low-grade inflammation, inflammaging and age-related disorders.
The aim of this collaborative project is to understand by which biological mechanisms perinatal or lifelong exposure to dietary CML contributes to the induction of chronic, low-grade inflammation and the occurrence of related chronic diseases. The novelty of this study lies in its interdisciplinary examination of the molecular mechanisms underlying the deleterious effects of CML, and its testing of antagonists that block the CML-mediated cellular response via RAGE, the receptor for AGEs.
Our strategy is to use transgenic mice, C. elegans and cellular models in order to fully elucidate the mechanisms by which CML acts in vivo (the CML–RAGE axis), and to develop a new anti inflammaging drug.
 ANR MIMETIC 2020-2021 (UdLille – CHULille)
ANR MIMETIC 2020-2021 (UdLille – CHULille)
The clinical spectrum of patients affected by coronavirus disease 2019 (COVID-19) ranges from asymptomatic to multiorgan failure requiring admission to the intensive care and resuscitation (ICU) unit. A better understanding of the physiopathological mechanisms is a prerequisite for the discovery of new therapeutic targets. In addition, the identification of early and predictive markers of patient development would help guide them to the best care unit and prevent ICU overload. The MIMETIC project will identify new mitochondrial and metabolic markers predictive of patient progress, allowing them to optimize their management and relieve UHIs. We hope to identify metabolic and mitochondrial parameters that will give a better understanding of the pathophysiology, to identify new therapeutic targets and to find new biomarkers that will allow an optimized management of patients suffering from COVID-19.
Members
Éric BOULANGER
PU-PH, group leader
ORCID number : 0000-0002-5204-2849
Sabine VENANT
University Department Assistant
Frédéric TESSIER
PU
ORCID number : 0000-0001-8096-5715
Mike HOWSAM (IGR)
IGR
ORCID number : 0000-0002-6344-8908
Sarahi JARAMILLO-ORTIZ
Post-doc
Matheus Thomaz NOGUEIRA SILVA LIMA
PhD student
Manon LAMBERT
M2R
Steve LANCEL
PU
ORCID number : 0000-0002-3292-5433
Marie FRIMAT
MCU-PH
ORCID number : 0000-0002-7461-8298
Raphaël FAVORY
PU-PH
ORCID number : 0000-0001-7772-2608
Marc LAMBERT
PU-PH
ORCID number : 0000-0002-3485-8238
Sébastien PREAU
PU-PH
ORCID number : 0000-0002-8638-7183
Cécile YELNIK
MCU-PH, PhD student
Yara SALEH
Post-doc
Amélie PAU
Tech
Gaëlle GROLAUX
Tech
Xingyu LU
M2R
Alexandre PIERRE
M2R
Chantal FRADIN
MCU
ORCID number : 0000-0002-3883-4401
Constance DUBOIS
PhD student
Aurélie MAILLIEZ
CCU-AH, PhD student
Charles PAUL CONSTANT
Tech
Numéro ORCID : 0000-0002-0554-5937
Stéphane RIANHA
M2R
Alina GHINET
E-C
ORCID number : 0000-0001-6468-4331
Christophe FURMAN
MCU
ORCID number : 0000-0003-4799-288X
Emmanuelle LIPKA
MCU
ORCID number : 0000-0003-2304-2214
Muriel BILLAMBOZ
E-C
ORCID number : 0000-0003-1486-7206
Lisa BONIN
PhD student
Audrey DAMIENS
PhD student
Théo GUERIN
PhD student
Georgiana NEGRU
PhD student
Christine SAFI
PhD student
Andreea ZUBAS
PhD student
Adrian-Sorin NICA
Tech
ORCID number : 0000-0002-8474-890X
Typhaine DESPRES
M2R
Antoine MORVAN
M2R
Publications
Guilbaud A, Howsam M, Niquet-Léridon C, Delguste F, Fremont M, Lestavel S, Maboudou P, Garat A, Schraen S, Onraed B, Foligné B, Boulanger É, Tessier FJ. (2020)
The Effect of Lactobacillus fermentum ME-3 Treatment on Glycation and Diabetes Complications.
Mol Nutr Food Res 64:e1901018.
Teissier T, Quersin V, Gnemmi V, Daroux M, Howsam M, Delguste F, Lemoine C, Fradin C, Schmidt AM, Cauffiez C, Brousseau T, Glowacki F, Tessier FJ, Boulanger E, Frimat M. (2019)
Knockout of receptor for advanced glycation end-products attenuates age-related renal lesions.
Aging Cell 18(2):e12850.
Yu Y, Wang L, Delguste F, Durand A, Guilbaud A, Rousselin C, Schmidt AM, Tessier F, Boulanger E, Neviere R. (2017)
Advanced glycation end products receptor RAGE controls myocardial dysfunction and oxidative stress in high-fat fed mice by sustaining mitochondrial dynamics and autophagy-lysosome pathway.
Free Radic Biol Med. 112:397-410.
Tessier FJ, Niquet-Léridon C, Jacolot P, Jouquand C, Genin M, Schmidt AM, Grossin N, Boulanger E. (2016)
Quantitative assessment of organ distribution of dietary protein-bound (13) C-labeled N(ε) -carboxymethyllysine after a chronic oral exposure in mice.
Mol Nutr Food Res 60:2446-2456.
Grossin N, Auger F, Niquet-Leridon C, Durieux N, Montaigne D, Schmidt AM, Susen S, Jacolot P, Beuscart JB, Tessier FJ, Boulanger E. (2015)
Dietary CML-enriched protein induces functional arterial aging in a RAGE-dependent manner in mice.
Mol Nutr Food Res 59:927-938.
Keywords
Glycation ; AGEs ; RAGE ; Inflammation ; Aging ; Inflammaging ; Mitochondria ; Transdisciplinary research ; Physiology ; Analytical chemistry ; Molecular biology ; Cell biology ; Drug design ; LC-MS/MS ; Nuclear magnetic resonance ; Micro-injection ; Binding test ; Chiral separation ; Vessels ; Kidney
Team contact
Pr Éric Boulanger
PU-PH, Lille University
eric.boulanger@univ-lille.fr
03 20 62 69 68
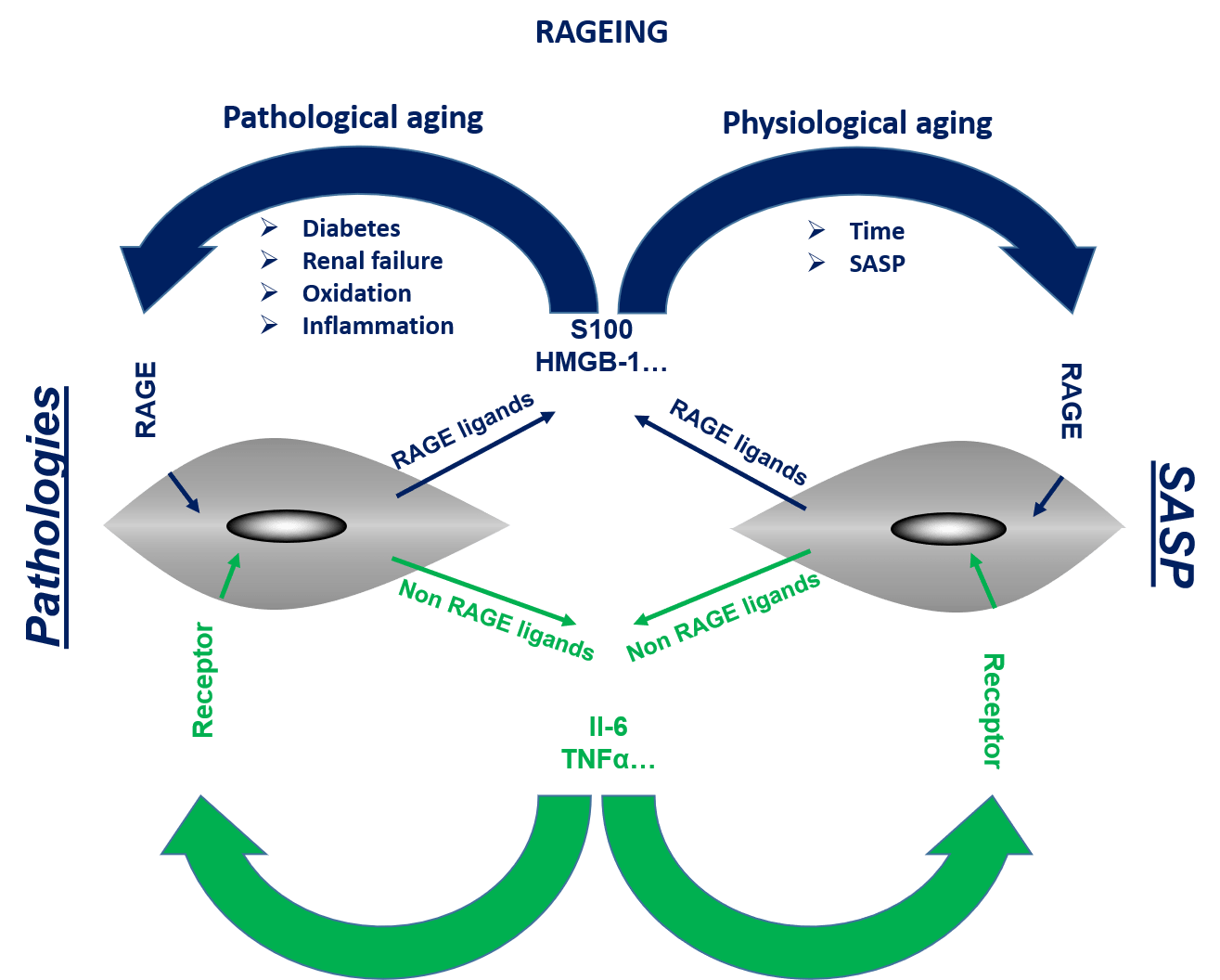
RAGEING : Implication de l’axe RAGE-ligand au cours de l’inflammaging.