Biology of Apicomplexa parasites : factors regulating growth differenciation and virulence
Presentation
The apicomplexan parasites Plasmodium and Toxoplasma gondii, causative agents of malaria and toxoplasmosis respectively, continue to present a public health menace and to weigh heavily on the socio-economic development in many regions of the world. Our current research is focusing on the following topics: 1) to understand the role of transcription factors and epigenetic regulation of gene expression in controlling essential pathways for proliferation and differentiation of T. gondii; 2) to examine reversible phosphorylation processes with particular emphasis on issues related to phosphatases and their regulation in Plasmodium and T. gondii and 3) to elucidate the molecular mechanisms that regulate the secretion of essential virulent factors by T. gondii and decipher their impact on the host immune responses. Finally, we explore in parallel target-based approaches for the discovery of novel anti-Plasmodium and T. gondii drugs.
Highlights
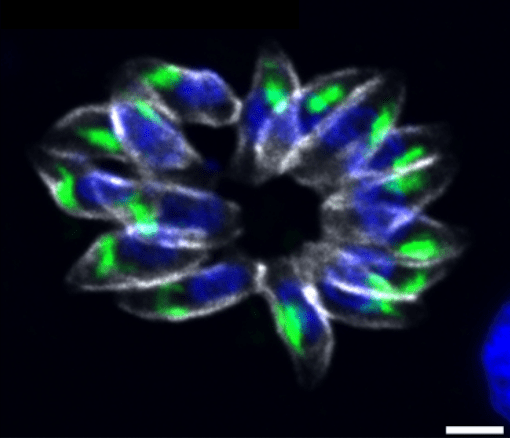
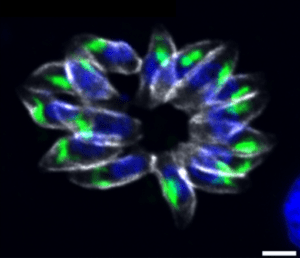
- Gene regulation: We have shown that the cooperation of two ApiAP2 transcription factors is crucial for the regulation of virulence factor expression in the apicomplex T. gondii parasite. We also demonstrated that another factor from the ApiAP2 family is a key regulator of division in this parasite. Indeed, it controls not only the formation of daughter parasites but also the expression of other transcription factors involved in the division cycle (Lesage KM et al. 2018, Khelifa AS et al 2021).
- Post translational modifications: Our studies on reversible phosphorylation phenomena in Plasmodium revealed that the regulators of Protein Phosphatase 1, a serine/threonine phosphatase, are as essential to the parasite as the catalytic subunit of the enzyme. In addition, interference with regulatory/catalytic subunit interactions leads to inhibition of P. falciparum growth in vitro (Hollin T et al. 2019, Gnangnon B 2019, Khalife J et al. 2021).
- Parasite effector secretion and host immune responses: We revealed the key role of the small GTPase TgRab11A in the secretion of dense granule proteins, which contain essential virulent factors enabling parasite survival into host cells. Moreover, we recently described that the induction of the host Unfolded Protein Response (UPR) in infected dendritic cells plays an essential protective against T. gondii infection (Venugopal K et 2020, Poncet A et al. 2021).
- Target-based drug discovery: A project on the search for new anti-parasitic drugs targeting epigenetic modifications (consortium A-ParaDDisE, project funded by Europe), allowed us to identify 3 new molecules as powerful anti-malarial drugs (Bouchut A et al. 2019).
Transversal projects
 How T. gondii influences infected neuron activity.
How T. gondii influences infected neuron activity.
T. gondii forms latent cyst in the brain of immunocompetent individuals. With no drug currently available to eliminate the latent cysts in the brain of infected hosts, it can influence the activity of neurons and may be implicated in neurobiological diseases affecting the elderly. T. gondii infection has therefore lifelong effects on the central nervous system of immunocompetent hosts. It has long been known that T. gondii influence the activity of neurons, but how this parasite manipulates the neuron and how the neuron respond to the infection remain to be elucidated. In close collaboration with Dr. JC Lambert’s Team (U1067), we investigate the direct impact of T. gondii infection on the neuron. This project was supported by the CPER CTRL 19.
 Impact of Toxoplasma-triggered neuro-inflammation and modulation of the kynurenine pathway on Tau pathology.
Impact of Toxoplasma-triggered neuro-inflammation and modulation of the kynurenine pathway on Tau pathology.
Latent cerebral toxoplasmosis is correlated with the establishment of a low-grade chronic inflammation of the brain characterized notably by the activation of immune resident cells, such as astrocytes and microglia. Upon activation, these cells trigger the kynurenine pathway and the generation of neurotoxic metabolites that can alter neuronal functions. In close collaboration with the lab of Dr. David Blum (UMR-S1172 “Alzheimer & Tauopathies”), which has developed a murine model of progressive tauopathy, our project aims to address whether latent cerebral toxoplasmosis modulates the KP and thereby exacerbates the progression of Tau pathology.
Members
Jamal KHALIFE
DR CNRS, group leader
ORCID number : 0000-0002-4359-8976
Sabrina MARION
MCU, Univ Lille
ORCID number : 0000-0001-7824-955X
Christine PIERROT
CR IPL
ORCID number : 0000-0002-6835-6789
Mathieu GISSOT
DR CNRS
ORCID number : 0000-0003-0047-8189
El Moukhtar ALIOUAT
Professor, Univ Lille
ORCID number : 0000-0003-2905-7126
Emmanuel ROGER
MCU, Univ Lille
ORCID number : 0000-0002-0982-0369
Engineer CE, IPL
Engineer CE, IPL
Engineer IE, CNRS
Engineer AI
PhD student
PhD student
Post-doc
PhD student
PhD student
Master 2 student, Univ Tours
Post-doc
Publications
Bouchut A et al. 2019.
Identification of novel quinazoline derivatives as potent antiplasmodial agents.
European Journal of Medicinal Chemistry; 161
Venugopal K et 2020.
Rab11A regulates dense granule transport and secretion during Toxoplasma gondii invasion of host cells and parasite replication.
PLoS pathogens; 16(5).
Khalife J et al. 2021.
The Multifaceted Role of Protein Phosphatase 1 in Plasmodium.
Trends in parasitology; 37 (2).
Khelifa AS et al 2021.
TgAP2IX-5 is a key transcriptional regulator of the asexual cell cycle division in Toxoplasma gondii.
Nature Communications; 12 (116).
Poncet A et al. 2021.
The UPR sensor IRE1α promotes dendritic cell responses to control Toxoplasma gondii infection.
EMBO Reports In press.
Keywords
Team contact
Jamal Khalife
Group leader
jamal.khalife@pasteur-lille.fr
03 20 87 79 68
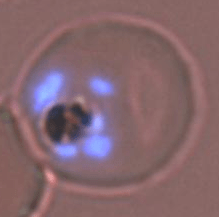
Red blood cell infected with P. falciparum.